Molecular sieve adsorption
Overview: Molecular sieve is a type of aluminosilicate, mainly composed of silicon and aluminum connected by oxygen bridges to form an open skeleton structure. There are many uniformly sized pores and neatly arranged cavities with a large internal surface area in the structure. In addition, it also contains metal ions with lower electricity prices and larger ionic radii, as well as water in its chemical state. Due to the continuous loss of water molecules after heating, but the crystal skeleton structure remains unchanged, many cavities of the same size are formed. The cavities are connected by many micropores of the same diameter, and molecules smaller than the pore diameter are adsorbed inside the cavity, while molecules larger than the pore diameter are repelled outside, causing molecules of different sizes and shapes to separate until they are screened, hence the name molecular sieve.
Molecular sieves are mainly used for deep drying of various gases and liquids, separation and purification of gases and liquids, catalyst carriers, etc
It refers to a type of substance with uniform micropores, whose pore size is equivalent to that of ordinary molecules. Molecular sieves have a wide range of applications, including efficient desiccants, selective adsorbents, catalysts, ion exchangers, etc. However, the cost of synthesizing molecular sieves using chemical raw materials is high. The commonly used molecular sieves are crystalline silicates or aluminosilicates, which are connected by oxygen bridge bonds between silicon oxygen tetrahedra or aluminum oxygen tetrahedra to form pore and cavity systems with molecular sizes (usually 0.3~2 nm). They have the ability to screen fluid molecules of different sizes due to the different sizes and shapes of adsorbed molecules.
Function: It can distinguish molecules with different particle sizes, as well as adsorbed unsaturated molecules and polar molecules.
Working principle:
Initial adsorption function: The adsorption of substances by molecular sieves comes from physical adsorption (van der Waals force), and their crystal pores have strong polarity and Coulomb field, exhibiting strong adsorption ability for polar molecules (such as water) and unsaturated molecules. Screening function: The pore size distribution of molecular sieves is very uniform, and only substances with a molecular diameter smaller than the pore diameter can enter the crystal pores of the molecular sieve. By distinguishing molecules of different substances based on their adsorption priority and size, they are vividly called "molecular sieves".
Structure:
The protein polysaccharide polymer formed by this twists and turns, forming a sieve like structure with multiple micropores, which is called a molecular sieve. Molecular sieves only allow substances smaller than their micropores to pass through, and have a barrier effect on large molecules, bacteria, and other substances larger than their micropores. Make the matrix a defense barrier that restricts the spread of harmful substances such as bacteria.
Application types of zeolite molecular sieves
Zeolite molecular sieves are mainly applied in the treatment of VOCs in the form of disc rotors, cylindrical rotors, and fixed bed molecular sieves.
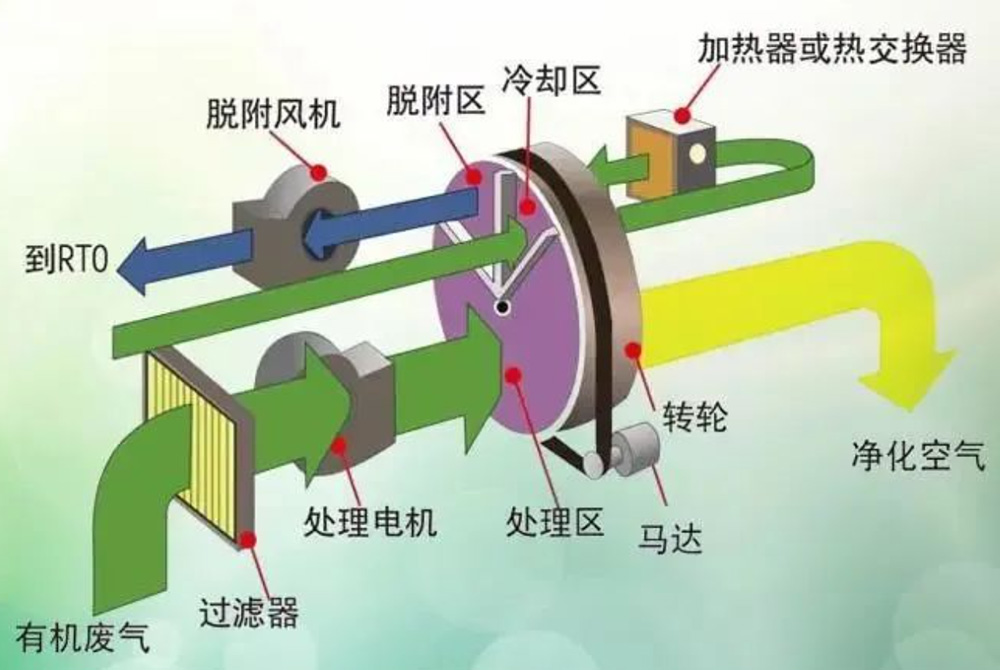
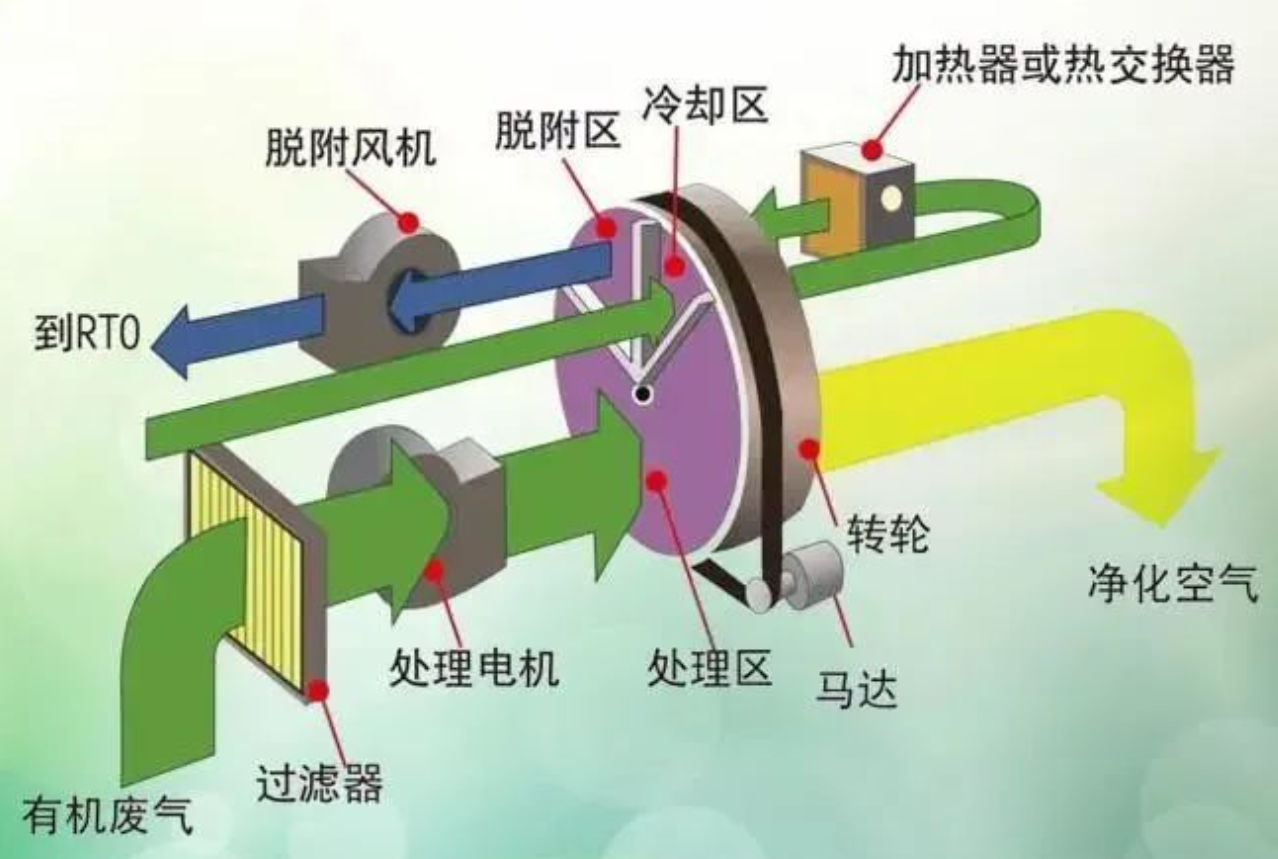
1) Disc wheel
The disc zeolite wheel structure is divided into three parts: adsorption zone, regeneration zone, and cooling zone, generally in a ratio of 10:1:1.
Adsorption zone: On the windward side of the exhaust gas, the wind speed is generally controlled at 2-4Nm/s, and the purification efficiency is generally between 90-97%. The purified gas enters the chimney.
Cooling zone: Generally, process gas is used for cooling, which not only reduces the temperature of the zeolite in the cooling zone, making it adsorbent, but also smoothly achieves heat recovery.
Desorption zone: The gas that has undergone heat recovery is heated to a temperature of around 200 ℃ before entering the desorption zone for cooling. The air volume for desorption regeneration is generally 10-20 times that of the intake air. When conducting high-temperature regeneration, the maximum temperature can reach around 300 ℃, and it is necessary to choose a high-temperature regeneration type wheel during design.
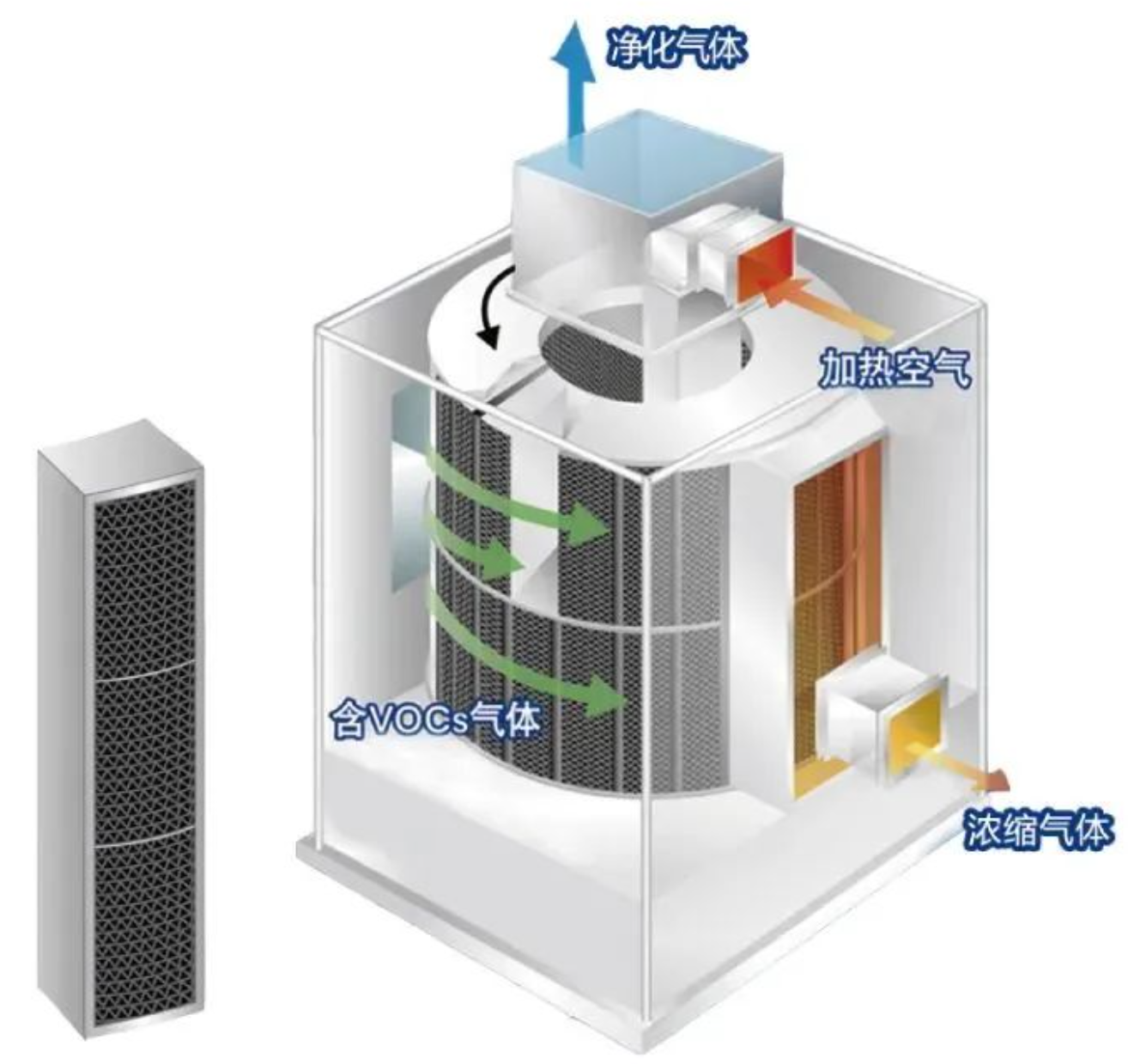
2)Cylindrical wheel
In addition to differences in material processing, the cylindrical zeolite wheel has significant structural differences from the disc type. Modular zeolite materials are used to fill the cylindrical structure to achieve VOCs adsorption and concentration. Without the disc cooling zone on the structural partition, the desorption energy consumption is higher than that of the disc wheel when the concentration ratio is the same. The concentration ratio of the cylindrical wheel can reach up to 50 times, and the higher the concentration ratio, the smaller the specifications of the combustion device or other equipment it is matched with. Its modular zeolite is also a major feature of the equipment, which is easy to install and maintain.
3)Fixed bed zeolite molecular sieve
Whether it is a cylindrical wheel or a disc wheel, their application scenarios require continuous working conditions. Therefore, based on the development of fixed bed activated carbon, a fixed bed honeycomb zeolite molecular sieve adsorption bed was formed by changing the adsorption material.
Due to the characteristics of zeolite molecular sieves, their dynamic adsorption capacity is much smaller than that of activated carbon, and their desorption temperature is high (around 250 ℃). The use of a fixed bed system increases the complexity of the system in terms of valve airtightness, insulation of the fixed bed, and control of desorption temperature rise. The application of fixed bed zeolite systems still requires the accumulation of experience and optimization of difficult solutions.